Solutions for parenteral use were historically the first dosage form, the development and production of which was mastered by YURіA-PHARM. At present, drugs in liquid dosage form remain the basis of our product portfolio. This specialization allows us to pay special attention to the development of modern technologies for the production of parenteral solutions, and to ensure maximum product quality through its in-depth research and comprehension.
On one hand, direct administration of drugs into the bloodstream ensures maximum bioavailability, as well as rapid achievement of effect, in contrast to other dosage forms. On the other hand, microorganisms or products of their metabolism, as well as various impurities of organic and inorganic nature, may enter the human body along with the solution of the drug substance. That is why quality assurance is the main focus in the development and production of parenteral solutions. For this purpose, sterility, the presence of endotoxins and pyrogens, the content of heavy metals and impurities formed by the degradation of the active substance or extracted into the solution from packaging materials, are carefully checked at various production stages.
At the same time, taking into account that any control is selective and cannot guarantee the quality of all manufactured units, we are actively implementing the Quality by Design approach. This allows us to minimize the very possibility of deviations in the technological process and exclude the possibility of obtaining a low-quality product through special tools for risk analysis, drug development and organization of their production.

Solutions for infusions and injections
The production technology of drugs in the form of solutions for infusion and injection has been optimised by pharmaceutical companies around the world for many decades and is, in fact, classic. The process starts with dissolving the active substance in water, prepared according to strict standards of pharmacopeia. YURіA-PHARM pays special attention to water quality, as it comprises the main component of the drug (usually more than 95%). In addition to the active substance, various auxiliaries are dissolved in water, to provide stability and ensure physiological values of pH and osmolarity of the solution.
The prepared solution is poured into vials or ampoules made of glass or inert plastic, sealed and sterilized. The standard sterilization regimen involves heating the drug to 121 ⁰C for 15 minutes. At such temperatures, bacteria, viruses or fungi loose viability, which guarantees the sterility of the final product.

Emulsions for parenteral administration
Some active substances that need to be injected are insoluble in water. Emulsions for parenteral use are one of the optimal dosage forms for such drugs.
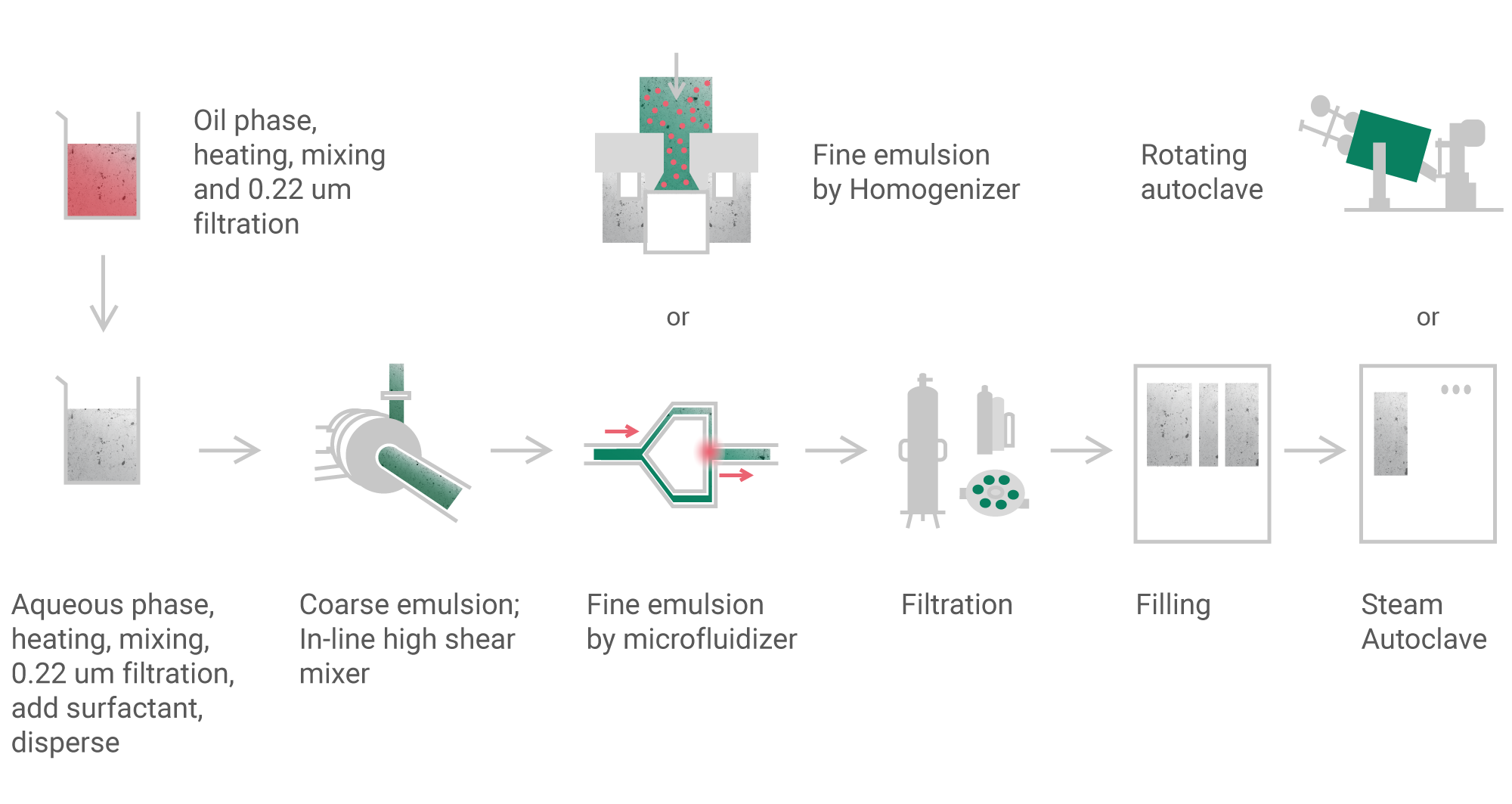
The main technology we use in the development of emulsions, involves dissolution of the hydrophobic active ingredient in highly purified vegetable oil. Aqueous buffer solution with physiological pH and osmolarity is prepared in a separate reactor. Oil with the dissolved active substance is emulsified in a buffer solution. The size of fat droplets is not more than 5-10 microns, which minimizes the risk of embolic complications in patients. Stability of emulsions is ensured by the introduction of surfactants into their composition which protect fat droplets from adhesion or sedimentation. As with other parenteral drugs, emulsions must be sterilized.
YURіA-PHARM R&D laboratories are fully equipped equipped for performing emulsion development with a rotor-stator homogenizer, autoclave, tools for measuring the size and charge of fat particles.