The first data on international multicenter clinical study RheoSTAT on the efficacy and safety of Rheosorbilact® infusion in therapy of pneumonia
Adequate and effective treatment of severe pneumonia is especially relevant in present situation. The most problematic issue is infusion therapy. The current evidence and guidelines recommend balanced crystalloid infusion for patients with severe pneumonia and sepsis. The composition of Rheosorbilact ® provides significant benefits in patients with severe infections, including respiratory infections.
According to the results of the randomized open blinded end-point RheoSTAT-CP0698 study, administration of Rheosorbilact® to patients with pneumonia (intravenous infusion at a dose of 200-400 ml/day for 3 days) effectively improves the clinical condition, reduces the manifestations of (multi-) organ failure and endogenous intoxication. Small-volume infusion therapy promotes rapid normalization of circulating blood volume, stabilization of hemodynamics, acid-base, electrolyte and gas composition of the blood, significantly improves saturation and reduces tachypnea.
The positive effect of therapy on renal function and inflammation has also been established. This therapy had a favorable safety profile (e. g., it did not lead to fluid overload, pulmonary edema, pleural effusion or other serious side effects, and was not associated with a clinically significant increase in endogenous serum lactate level). The RheoSTAT-CP0698 study substantiates the feasibility of using Rheosorbilact ® in the complex treatment of pneumonia.
KEY WORDS: pneumonia, infusion therapy, efficacy, safety, Rheosorbilact.
Conflict of interests: none.
Despite significant advance in diagnosis and treatment, the mortality rate due to pneumonia has not changed significantly over the past 30 years [1]. 5 to 15 % of hospitalized patients die within 30 days [2], and mortality rate in intensive care units (ICU) reaches 17-48 % [3]. Adequate and effective treatment of severe pneumonia is especially relevant in present conditions, and the most challenging issue is infusion therapy. For the most part, fever and more intense perspiration are successfully managed by oral fluid administration.
However, it is not always possible in critical patients, which leads to hypovolemia. In addition, in response to bacterial exo- and endotoxins, as well as under the influence of endogenous cytokines and histamine, vasoplegic vasodilation associated with hypotension and septic shock occurs as pneumonia is the most common cause of sepsis [4].
According to a recent multicenter study, sepsis and septic shock complicate the course in a third of patients hospitalized due to pneumonia [5]. Systemic hypovolemia is also associated with the “capillary leakage” phenomenon caused by endothelial dysfunction and increased vascular permeability due to damaged glycocalyx. Endothelial glycocalyx is a matrix of membrane-bound glycoproteins and proteoglycans on the inner surface of endotheliocytes of 0.2 to 8 µm, retaining 700 to 1500 ml of intravascular fluid like a sponge [6].
The capillary glycocalyx layer acts as a semipermeable barrier that prevents penetration of large molecules, in particular albumin, through the gaps between endothelial cells. It is the glycocalyx that is responsible for establishing the hydrostatic pressure-resistant oncotic gradient. According to revision of the Starling principle, considering the endothelial glycocalyx model, an increase in plasma oncotic pressure resists fluid filtration from the intravascular to interstitial space, but does not cause fluid return back to the vessel [7].
Water from the extracellular matrix mostly returns to the intravascular space through the lymphatic system [8]. In severe infections and sepsis, tumor necrosis factor causes activation of nuclear factor-kB and endothelial damage, and lipopolysaccharides damage the glycocalyx by the mechanism of oxidative stress [9, 10]. Glycocalyx may be damaged by a number of chronic diseases, in particular by diabetes mellitus [11], and comorbidity is known to be one of the extrapulmonary factors that determine the severity of pneumonia.
As a result, the liquid part of the blood is moved to the interstitial extracellular space. At this stage, a vicious circle is triggered: oxygen transport in the lungs is disrupted, causing or deepening respiratory distress; hypovolemia and hypoperfusion of organs and tissues increases, causing or aggravating multiple organ failure [7, 12, 13]. In addition, intracellular edema disrupts a number of biochemical processes, such as glucose metabolism, cardiomyocyte contractility, inflammatory reactions, endogenous antimicrobial activity of plasma, etc. [7].
Under these conditions, intravenous infusion therapy is a basic pathogenetic treatment. Guidelines mainly focus on the etiotropic treatment of pneumonia, while the issues of pathogenetic therapy are only covered conceptually. Infusions are indicated to be combined with early respiratory support and strict monitoring of clinical and laboratory parameters, such as mean blood pressure, central venous pressure (CVP) and central venous blood saturation in IСU settings.
In septic hypotension, short-term initial liquid resuscitation is recommended with the predominant use of balanced crystalloid solutions and early administration of low or medium doses of vasopressors – epinephrine at initial dose of 0.2-0.5 µg/kg/min, in case of heart failure – norepinephrine or dobutamine [14]. This principle, known as “early goal-directed therapy” (EGDT), was introduced into clinical practice as early as 2001, after E. Rivers et al. demonstrated that optimizing hemodynamics in patients with septic shock (including 39 % with pneumonia) reduces hospital mortality by 16 % [15].
The following criteria for EGDT initiation were proposed:
- Inability to maintain an average blood pressure ≥65 mm Hg without administration of vasopressors;
- Serum lactate level ≥2 mmol/l (18 mg/dl) in the absence of hypovolemia;
- Quick SOFA score ≥2, i. e. the presence of at least two of the following signs: respiratory rate ≥22/min, systolic blood pressure ≤100 mm Hg, Glasgow Coma Scale score ≤14 [16].
After stabilization of hemodynamic parameters, i. e. reaching the mean blood pressure of 65 to 90 mm Hg or in the absence of shock, it is recommended to use a restrictive type of infusion therapy [14]. After all, excessive infusion volume can increase pulmonary edema and hypoxemia, is associated with an increase in the time spent in IСU or on mechanical ventilation, and a significantly higher risk of death [17-19]. It has also been shown that the volume of intravenous fluids administered by infusion is independently associated with the degree of glycocalyx degradation, which indicates the possibility of iatrogenic endothelial damage due to improper infusion therapy strategy [10].
Investigators hypothesize that intravenous fluid can cause direct damage and exfoliation of the endothelium, regardless of fluid balance [10]. In the presence of inflammatory mediators, sudden stretching of blood vessels caused by liquid boluses stimulates endothelial expression of metalloproteinases and promotes activation of cathepsin L and endothelial heparanase, which cause glycocalyx exfoliation. Infusion of isotonic solutions promotes the activation of circulating white blood cells and their release of elastase, which can also damage the glycocalyx [10].
In addition, fluid overload causes intra-abdominal hypertension with the compression of internal organs leading to their dysfunction [18, 20], as well as slows down the recovery of renal function or increases the risk of acute renal impairment [21-23].
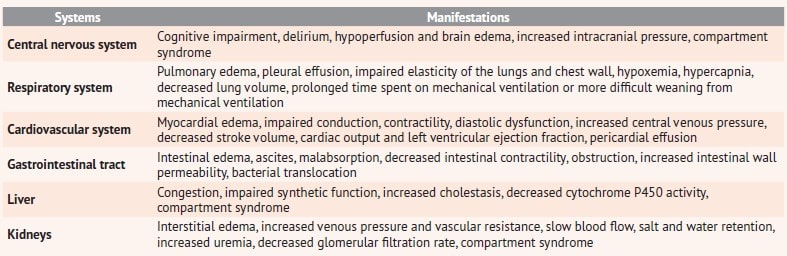
All negative impacts are summarized in table 1 [7, 12, 13].
Table 1. Consequences of fluid overload in infusion therapy
How shall clinicians balance benefits and risks? How to choose an adequate infusion solution? What is the evidence for the strategy and tactics of infusion therapy in patients with severe pneumonia? These questions support the feasibility of our study, which was aimed at finding evidence for infusion therapy in severe pneumonia.
Materials and methods
An electronic search in the PubMed, MEDLINE and Cochrane Library databases over the past 20 years was conducted using a sensitive strategy without language restrictions for the following keywords: “pneumonia”, “sepsis”, “septic shock”, “acute respiratory distress syndrome”, “hypoxemia”, “mortality”, “early targeted therapy”, “liquid therapy”, “liquid resuscitation”, “restrictive type of infusion therapy”, “choice of infusion solution”, “randomized controlled study”, “review”, “meta-analysis”.
For data on sepsis, septic shock, and acute respiratory distress syndrome, the proportion of patients with pneumonia was determined and only those studies where at least a third of patients had pneumonia were included.
The results of the recently completed international multicenter open-label blinded end-point randomized controlled phase III-IV RheoSTAT-CP0698 study (RCS) were also reviewed based on a report provided by “Yuria-Pharm”. The study was conducted from 1 September 2017 until 28 February 2020 by a contract research organization in accordance with the Good Clinical Practice (ICH GCP), ethical standards of the Helsinki Declaration of the World Medical Association and national standards, and included in the Cochrane Library [24], one of the most authoritative evidence-based medicine electronic databases, which indicates a high level of evidence.
Overall, the RheoSTAT-CP0698 RCS included 628 patients with sepsis, peritonitis, burn disease, and pneumonia who were treated in 44 clinical centers in 7 countries. The RheoSTAT-CP0698 pneumonia sub-study involved 150 patients from 12 clinical centers in 6 countries – Ukraine, Moldova, Georgia, Uzbekistan, Kazakhstan and Vietnam.
Essential inclusion criteria for the RheoSTAT-CP0698 pneumonia sub-study were: age 18-60 years, confirmed community-acquired pneumonia with PSI/PORT risk class IV or higher, provided that the period from the initiation of antibacterial therapy did not exceed 48 hours; signed informed consent to participate in the study; initial quick SOFA score ≥2; blood pH <7.45, blood potassium <5.1 mmol/l and blood sodium <145 mmol/l.
The average age of the study participants was 41.3 years (62 % male), including 33 % with concomitant diseases (12 % – arterial hypertension, 21 % – others). Patients were randomized to the treatment group (n=78) and control group (n=72). Subjects of the treatment group received Rheosorbilact® infusion solution for 3 days by intravenous infusion at a dose of 200-400 ml/day. On day 3, their efficacy criteria were evaluated, and after 14±2 days, safety and disease outcomes were monitored (fig. 1).
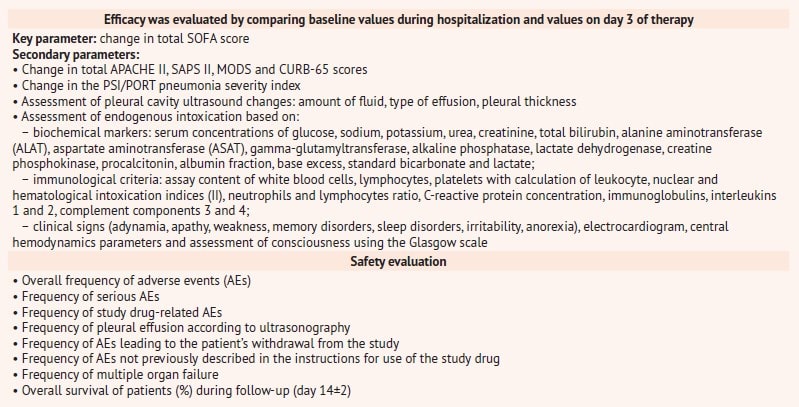
It is worth noting a thorough and objective evaluation of the efficacy and safety of the study drug, which was carried out on the basis of numerous evaluation scales and clinical and laboratory values indicated in table 2.
Fig. 1. RheoSTAT-CP0698 pneumonia study design scheme
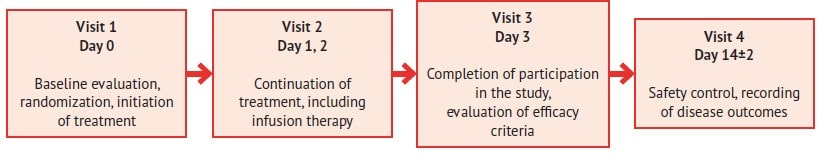
Table 2. Criteria for evaluating efficacy and safety in the RheoSTAT-CP0698 pneumonia study
Results and discussion
There are two main classes of infusion agents – colloids and crystalloids. Colloids include albumin, hydroxyethyl starch, and gelatin. Due to oncotic activity, colloids should theoretically slow down capillary leakage. However, in patients with severe infection, this effect is quite short-term due to glycocalyx damage [8, 13].
Compared to crystalloids, colloids have a slightly longer intravascular space elimination half-life, although capillary leakage affects both classes [25]. Other hypothetical benefits of colloids include an anti-inflammatory effect and the ability to absorb nitric oxide, but this only applies to albumin [26].
There are no major RCS that prove a clear difference in mortality between crystalloid or colloid infusion therapy in pneumonia or sepsis. The SAFE RCS was quite large and included critically ill adults comparing 0.9 % sodium chloride solution and albumin as liquid resuscitation agents. Despite the absence of a significant difference in 28-day mortality in the general group, better results were reported with albumin in patients with severe sepsis and acute respiratory distress syndrome, but worse in patients with severe traumatic brain injury [27, 28].
Hydroxyethyl starch solutions are associated with acute kidney injury in critically ill individuals, making them to be recognized as dangerous in the United States and Europe [29, 30]. Recent international guidelines for the management of sepsis do not recommend the use of colloids as a starting solution for liquid resuscitation due to lack of benefits and excessive costs [31].
Among the crystalloids, non-buffer solutions (isotonic sodium chloride solution) and buffer multi-electrolyte solutions can be distinguished, the latter differing in their composition, chloride concentration, pH and osmolarity, but being closer to plasma than isotonic sodium chloride solution. Resuscitation using 0.9 % sodium chloride solution is associated with the occurrence of hyperchloremic metabolic acidosis, acute kidney injury and dangerous vital organ dysfunction [32-35].
Despite this, isotonic sodium chloride solution remains the most commonly used crystalloid solution [36], which is also most often used as a solvent for intravenous administration of various drugs [33].
Two recent RCS, SALT-ED and SMART, indicate clear advantages of balanced buffer solutions over isotonic sodium chloride solution. Although there were no differences in short-term mortality, administration of 0.9 % sodium chloride solution was associated with a higher risk of acute kidney injury, including death, the need for dialysis, or long-term renal impairment [34, 35].
Special attention should be given to infusion solutions containing polyatomic alcohols, primarily sorbitol, which has a number of advantages:
- Due to its slow conversion to monosaccharides, it is utilized better than glucose, and does not cause carbohydrate overload;
- After administration, it is quickly incorporated into the general metabolism (80 % is utilized by the liver, 5 % is deposited in brain tissues, myocardium and skeletal muscles, the rest is excreted in the urine or used for urgent energy needs);
- Eliminates intestinal spasm caused by acetylcholine, stimulates peristalsis without acute increasing, which substantiates its use in the postoperative period;
- In hypertonic concentration, has a significant anti- edematous action, in particular promotes the reverse development of pulmonary edema, is characterized by an osmotic diuretic effect, which is important in oligoanuria and acute kidney injury;
- Due to powerful cholecystokinetic and choleretic action, facilitates restoration of normal digestive function, has a proven therapeutic effect in acute and chronic hepatitis and toxic liver injury;
- In isotonic concentration, acts as a disaggregant, improving microcirculation and tissue perfusion.
Among sorbitol-containing products, it is worth noting Rheosorbilact®, a complex polyfunctional infusion product manufactured by “Yuria-Pharm” (Ukraine). In addition to sorbitol, it contains other important electrolytes – potassium, calcium and magnesium, but the chloride content of as much as 112.7 mmol/l reduces the risk of hyperchloremic acidosis.
Another important component of Rheosorbilact® is sodium lactate, which provides an alkalizing effect, increases the reserve and titrated alkalinity of the blood, corrects metabolic acidosis, which often complicates severe infections, sepsis, peritonitis, intestinal obstruction, renal failure, burns, shock, chronic hypoxia, etc.
It has a positive effect on the cardiac function, regeneration and respiratory function of the blood, stimulates the functions of the mononuclear phagocyte system, has a detoxification effect, increases diuresis, improves kidney and liver function. The concentration of sodium lactate in Rheosorbilact® is 5-6 times higher (160-180 mmol/l) than in most solutions for infusion, which provides a powerful therapeutic effect.
The presence of two agents with a synergistic detoxification effect and the ability to correct the acid-base and water-electrolyte balance puts this medicinal product on a par with the most powerful detoxification agents [37].
The successful use of Rheosorbilact® for detoxification and normalization of blood rheology in patients with severe purulent-inflammatory diseases such as peritonitis [38], destructive pancreatitis [39], diabetic foot syndrome [40] suggests an improvement in clinical outcomes of pneumonia. In addition, one of the clinical studies found that the administration of Rheosorbilact® in patients with pneumonia contributes to early normalization of body temperature, disappearance of astheno-vegetative syndrome manifestations and reduction the average length of stay in hospital, stabilization of the acid-base status and coagulogram values [41].
In addition, Rheosorbilact® has been studied in the RheoSTAT-CP0698 RCS, which provides a high level of evidence in patients with pneumonia. According to the study results, administration of Rheosorbilact® by intravenous infusion at a dose of 200-400 ml/day effectively improves the clinical condition, reduces the manifestations of (multi-) organ failure and endogenous intoxication in most of the analysed indications.
On day 3 of therapy, most patients had normalized body temperature, respiratory rate, blood saturation and gas composition, renal function, which significantly improved the score of all the scales used in the study for assessment of the severity of pneumonia and critical conditions (table 3).
Table 3. Parameters for evaluating the efficacy of Rheosorbilact® before and after therapy* 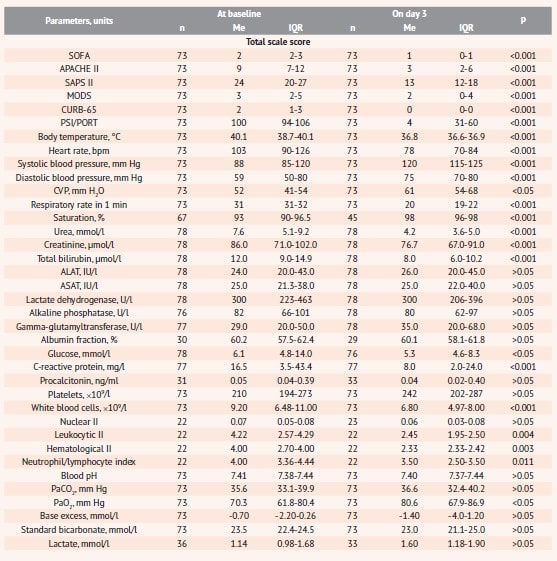
Notes: * data provided by “Yuria-Pharm” in the RheoSTAT-CP0698 RCS results report; n – number of observations; Me (IQR) – the median (interquartile range).
Small-volume infusion therapy with Rheosorbilact® provides an increase in the circulating blood volume, which is indicated by a significant increase in CVP, and stabilization of blood pressure and heart rate. On the other hand, after a 3-day course of infusions, CVP values did not increase to critically high levels (table 3), which, according to study data, are associated with an unfavorable prognosis and a higher risk of death [42].
This therapy allowed to reduce the total volume of infusion required to achieve a therapeutic effect without the risk of hyperhydration and fluid overload, which is especially important in older patients with comorbidity or in critical conditions that have a particularly unfavorable prognosis in pneumonia [1, 7, 12, 13].
The administration of Rheosorbilact® in complex therapy contributed to a significant decrease in the level of leukocytes, leukocyte and hematological II and normalization of the neutrophil/lymphocyte ratio, which indicates the ability to reduce the manifestations of infection-related endogenous intoxication. A significant decrease in C-reactive protein and a certain improvement in acid-base balance indicators were observed (table 3).
As for the safety profile, a total of 296 adverse events were reported in the treatment group in 43.6 % of patients, which was not statistically different from the control group (304 AEs in 50.0 % of patients). Most of the abnormalities were not clinically significant, and no serious adverse effects were reported during the study. No new safety signals have been received for the study drug. According to the analysis results, Rheosorbilact® has a favorable safety profile.
Exogenous lactate in the composition of Rheosorbilact® does not affect the level of endogenous lactate (table 3), elevation of which is associated with an unfavorable prognosis in sepsis and pneumonia [16, 43]. This proves the safety of the solution administered to patients with pneumonia. It should be noted that on day 3 of therapy, subjects of the treatment group showed a decrease in the percentage of laboratory abnormalities in the function of elimination organs, blood glucose and electrolyte levels, including clinically significant ones (fig. 2).
No cases of pulmonary edema or pleural effusion were detected in patients of the treatment group after infusion therapy (table 4).
Fig. 2. Percentage of abnormalities in the function of elimination organs, blood glucose and electrolytes before and after a 3-day treatment with Rheosorbilact®
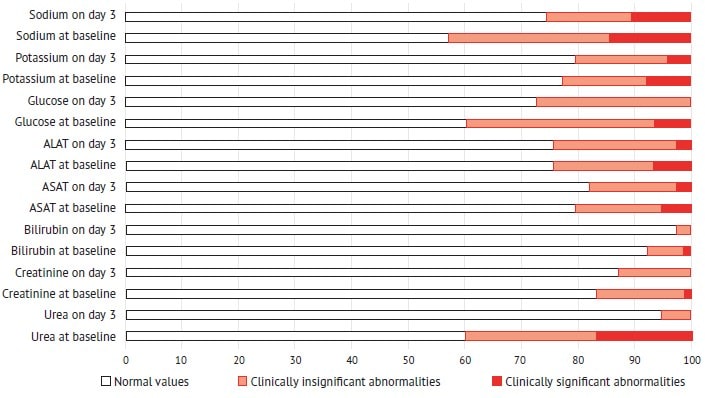
Table 4. Number of patients with reported fluid confirmed by ultrasound (safety population)

Conclusions
The current evidence and guidelines recommend the balanced crystalloid infusion as a pathogenetic therapy for severe pneumonia and sepsis. The composition of Rheosorbilact® provides significant benefits in patients with severe infections, including respiratory infections.
According to the results of the open blinded end-point RheoSTAT-CP0698 RCS, administration of Rheosorbilact® to patients with pneumonia (intravenous infusion at a dose of 200-400 ml/day for 3 days) effectively improves the clinical condition, reduces the manifestations of (multi-) organ failure and endogenous intoxication. Small-volume infusion therapy by Rheosorbilact promotes rapid normalization of blood volume circulating, stabilization of hemodynamics, acid-base, electrolyte and gas composition of the blood, significantly improves saturation and reduces tachypnea.
The positive effect of therapy on renal function and inflammation has also been established. This therapy had a favorable safety profile (e. g., it did not lead to fluid overload, pulmonary edema, pleural effusion or other serious side effects, and was not associated with a clinically significant increasing the blood levels of endogenous lactate). The RheoSTAT-CP0698 study substantiates the feasibility of using Rheosorbilact® in the complex treatment of pneumonia.
Authors:
Y.I. Feshchenko1, S. Beridze2, Dinh Thi Hoa3, V.Y. Molodtsov4, M.I. Gumeniuk1, N. Gogoreliani5, H.I. Sattarov6, N. Emukhvari7, G. Lupu8, Y.M. Mostovoi9, L.M. Kuryk1, Nguyen Thi Thu Anh10
1 National Institute of Phthisiology and Pulmonology named after F.G. Yanovsky of the NAMS of Ukraine, Kyiv, Ukraine
2 JSC “EVEX Medical Corporation” / Batumi State University named after Sh. Rustaveli, Georgia
3 198 Hospital, Hanoi, Vietnam
4 City Hospital № 1, Mykolaiv, Ukraine
5 JSC “EVEX Medical Corporation” / Kutaisi Referral Hospital, Georgia
6 Republican Scientific Center of Emergency Medical Aid, Tashkent, Uzbekistan
7 “Israel Georgian Medical Research Clinic HELSI Core LLC” LTD, Tbilisi, Georgia
8 Municipal Clinical Hospital “Sfinta Treime”, Chisinau, Moldova
9 Vinnytsia National Medical University / City Clinical Hospital № 1, Vinnytsia, Ukraine
10 Thai Binh University of Medicine and Pharmacy, Thai Binh, Vietnam
Literature:
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis.2018 Nov; 18 (11): 1191-1210. doi: 10.1016/S1473-3099(18)30310-4.
- Chalmers J. , Campling J. , Ellsbury G. , Hawkey P. , Madhava H. , Slack M. Community-acquired pneumonia in the United Kingdom: a call to action. Pneumonia (Nathan).2017 Oct 5; 9: 15.
- Phua J., Dean N.C., Guo Q., Kuan W.S., Lim H.F., Lim T.K. Severe community-acquired pneumonia: timely management measures in the first 24 hours. Crit. Care. 2016; 20 (1): 237. Published 2016 Aug 28. doi: 10.1186/s13054-016-1414-2.
- Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med.2001; 29: 1303-1310. doi: 10.1097/00003246-200107000-00002.
- Montull B., Menendez R., Torres A., Reyes S., Mendez R., Zalacain R. et al. Predictors of severe sepsis among patients hospitalized for community-acquired pneumonia. PLoS One.2016; 11: e0145929. doi: 10.1371/journal. pone.0145929.
- Woodcock T.E., Woodcock T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescrib-ing intravenous fluid therapy. Br. J. Anaesth.2012; 108 (3): 384-394.
- Chang R., Holcomb J. Choice of fluid therapy in the initial management of sepsis, severe sepsis, and septic shock. Shock. 2016 Jul; 46 (1): 17-26. doi: 10.1097/SHK.0000000000000577.
- Best M.W., Jabaley C.S. Fluid management in septic shock: a review of phys-iology, goal-directed therapy, fluid dose, and selection. Curr. Anesthesiol. Rep. 2019; 9: 151-157. https://doi.org/10.1007/s40140-019-00330-3.
- Liang Y., Li X., Zhang X., Li Z., Wang L., Sun Y., Liu Z., Ma X. Elevated levels of plasma TNF-αare associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock.2014; 41 (4): 275-281.
- Hippensteel J.A., Uchimido R., Tyler P.D., Burke R.C., Han X., Zhang F. et al. Intravenous fluid resuscitation is associated with septic endothelial gly-cocalyx degradation. Crit. Care. 2019; 23 (259). https://doi.org/10.1186/ s13054-019-2534-2.
- Nieuwdorp M., Mooij H.L., Kroon J., Atasever B., Spaan J.A., Ince C., Holleman F., Diamant M., Heine R.J., Hoekstra J.B. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006; 55 (4): 1127-1132.
- Malbrain M.L.N.G., Van Regenmortel N., Saugel B., De Tavernier B., Van Gaal P.J., Joannes-Boyau O., Teboul J.L., Rice T.W., Mythen M., Monnet X. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care. 2018 May 22; 8 (1): 66. doi: 10.1186/s13613-018-0402-x.
- Marik P. , Bellomo R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth.2016; 116 (3): 339.
- Adapted evidence-based clinical guideline. Nosocomial pneumonia in adults: etiology, pathogenesis, classification, diagnosis, antimicrobial therapy and prevention. Compilers: Feshchenko Y.I., Belosludtseva K.O., Golubovska O.A. et al. – Kyiv: National Academy of Medical Sciences of Ukraine, 2019. – 94 p.
- Rivers E., Nguyen B., Havstad S., Ressler J., Muzzin A., Knoblich B. et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med.2001; 345: 1368-1377. doi: 10.1056/NEJMoa010307.
- Singer M., Deutschman C., Seymour C., Shankar-Hari M., Annane D., Bauer M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock(Sepsis-3). JAMA. 2016; 315: 801-10.
- Koonrangsesomboon W., Khwannimit B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J. Crit. Care Med. 2015 Dec; 19 (12): 708-13.
- Cordemans C., De Laet I., Van Regenmortel N., Schoonheydt K., Dits H., Huber W. et al. Fluid management in critically ill patients: the role of ex-travascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann. Intensive Care. 2012; 2: S1.
- National Heart, Lung, and Blood Institute Acute Respiratory Distress Syn-drome (ARDS) Clinical Trials Network. Wiedemann H.P., Wheeler A.P., Ber-nard G.R. et al. Comparison of two fluid-management strategies in acute lung injury. N. Engl. J. Med. 2006; 354: 2564-75.
- Malbrain M.L., Marik P.E., Witters I., Cordemans C., Kirkpatrick A.W., Roberts D.J. et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014; 46: 361-80.
- Heung M., Wolfgram D.F., Kommareddi M., Hu Y., Song P.X., Ojo A.O. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury.Nephrol. Dial. Transplant. 2012; 27: 956-61
- Bouchard J. , Soroko S.B. , Chertow G.M. , Himmelfarb J. , Ikizler T.A. , Pa-ganini E.P. et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009; 76: 422-7.
- Stein A., de Souza L.V., Belettini C.R., Menegazzo W.R., Viégas J.R., Costa Pereira E.M. et al. Fluid overload and changes in serum creatinine after cardiac surgery: predictors of mortality and longer intensive care stay. A prospective cohort study. Crit. Care.2012; 16: R99.
- Efficacy and Safety of Rheosorbilact® Solution for Infusion, in a Complex Therapy of Pneumonia. NCT03824457. Cochrane Central Register of Cont-rolled Trials (CENTRAL). 2019. Issue 3. Available at: https://clinicaltrials.gov/ show/NCT03824457.
- Hahn R., Lyons G. The half-life of infusion fluids: an educational review. Eur. J. Anaesthesiol.2016; 33 (7): 475-82.
- Caironi P., Tognoni G., Masson S., Fumagalli R., Pesenti A., Romero M. et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med.2014; 370 (15): 1412-21.
- Finfer S., Bellomo R., Boyce N., French J., Myburgh J., Norton R. et al. A com-parison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004; 350 (22): 2247-56.
- SAFE Study Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J., Cooper D., Finfer S., Bellomo R., Norton R., Bishop N., Kai Lo S., Vallance S. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N. Engl. J. Med.2007 Aug 30; 357 (9): 874-84. doi: 10.1056/NEJMoa067514.
- Myburgh J., Finfer S., Bellomo R., Billot L., Cass A., Gattas D. et al. Hydrox-yethyl starch or saline for fluid resuscitation in intensive care. N. Engl. J. Med. 2012; 367 (20): 1901-11.
- Perner A., Haase N., Guttormsen A., Tenhunen J., Klemenzson G., Åneman A. et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N. Engl. J. Med.2012; 367 (2): 124-34.
- Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit. Care Med.2017; 45 (3): 486-552.
- MacDonald N. , Pearse R. Are we close to the ideal intravenous fluid? Br. J. Anaesth. 2017; 119 (suppl. 1): i63-71.
- Magee C.A., Bastin M.L.T., Laine M.E., Bissell B.D., Howington G.T., Moran P.R. et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study.Crit. Care Med. 2018; 46 (8): 1217-23.
- Self W.H., Semler M.W., Wanderer J.P., Wang L., Byrne D.W., Collins S.P. et al. Balanced crystalloids versus saline in noncritically ill adults. N. Engl. J. Med. 2018; 378 (9): 819-28.
- Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W. et al. Balanced crystalloids versus saline in critically ill adults. N. Engl. J. Med. 2018; 378 (9): 829-39.
- Dalton C. Why did sterile salt water become the IV fluid of choice? NPR. 2018. Available at: https: //www.npr.org/sections/health-shots/2018/03/31/597666140/why-did-sterile-salt-water-become-the-iv-fluid-of-choice. Accessed: 2/4/2018.
- Kondratsky B., Novak V. Experience of application in clinical practice of a complex infusion drug Rheosorbilact. The Art of Healing.2006; 1: 34-36.
- Khamidov D.B., Kosimov Z.K., Khomidov D.D., Kiyamov S.E. Rheosorbilact complex polyfunctional solution in intensive care of endogenous intoxica-tion in patients with acute peritonitis. Scientific and Practical Journal TIPPMK. 2011; 2: 77-79.
- Aliev N.A., Bobiev A.B., Khamidov D.B., Barotov E.D., Buriev T.N., Kurbanov D.A. et al. Rheosorbilact and Latren in the correction of endogenous intoxication and oxidative stress in patients with acute destructive pancreatitis. Emer-gency Medicine.2015; 64 (1): 57-59.
- Pronichev V., Styazhkina S., Mikhailov A. Efficiency of treatment with Rheo-sorbilact in patients with diabetic foot syndrome. Health, Demography, Ecology of the Finno-Ugric Peoples. 2016; 2: 30-32.
- Ryzhko O.O. Rheosorbilact infusion therapy. Ukr. Pulmonology Journal.2002; 1: 94-96.
- Semler M.W., Wheeler A.P., Thompson B.T. et al. Impact of initial central ve-nous pressure on outcomes of conservative versus liberal fluid management in acute respiratory distress syndrome.Crit. Care Med.2016; 44 (4): 782-789.
- Demirel B. Lactate levels and pneumonia severity index are good predic-tors of in-hospital mortality in pneumonia. Clin. Respir. J. 2018 Mar; 12 (3): 991-995.