Endothelitis in the pathogenesis of chronic CHD: clinical manifestations and treatment approaches

Abstract. Despite the fact that strategies to optimize the prevention and treatment of coronary heart disease (CHD) have been actively implemented in clinical practice in recent years, the consequences of this condition still represent a significant burden on human health, holding leading positions in the structure of mortality and morbidity.
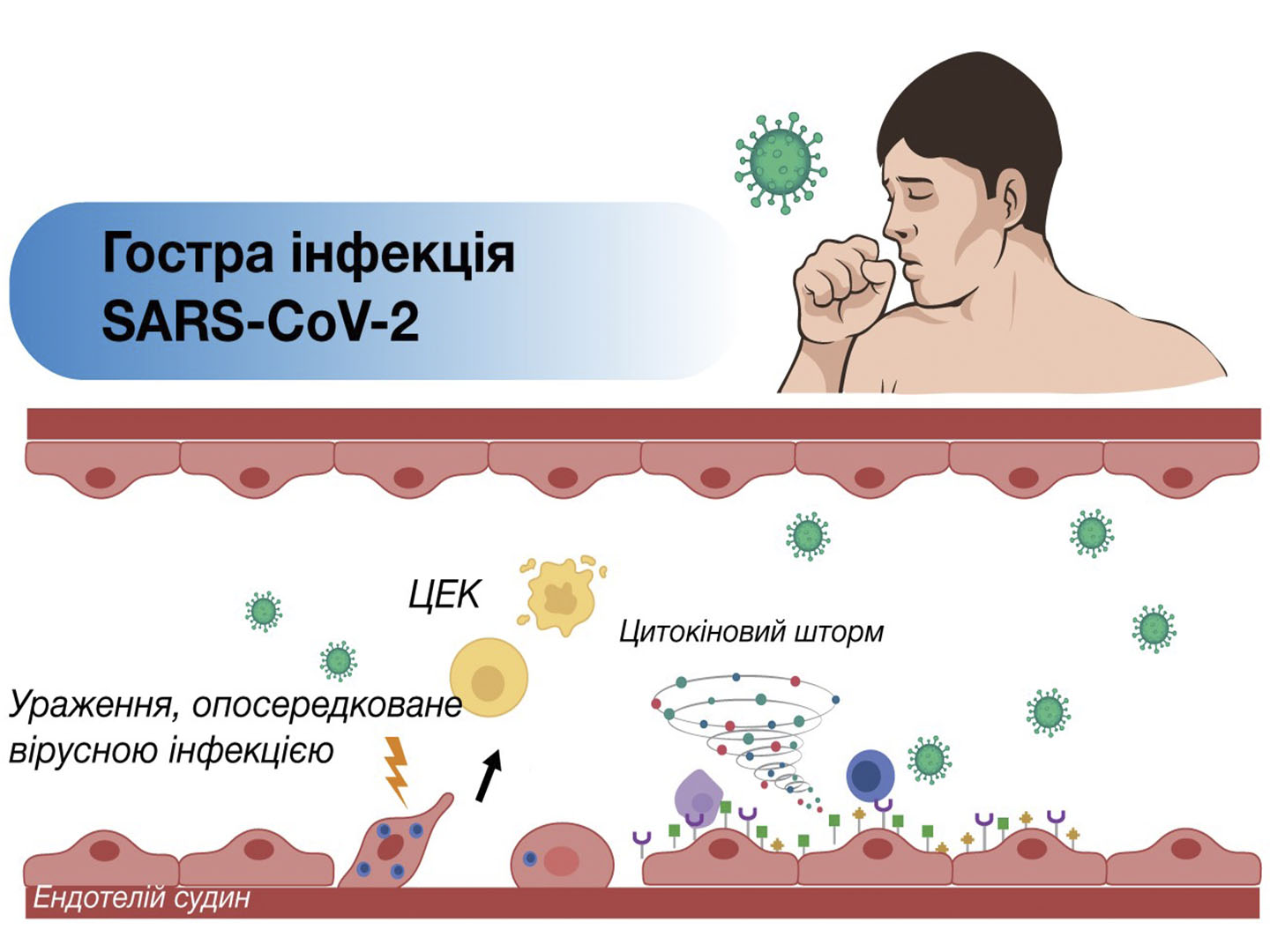
The etiology of myocardial ischemia is currently being studied comprehensively. Clinical, angiographic and autopsy data indicate a complex pathophysiology of CHD that goes beyond the generally accepted and simplified role of atherosclerosis. In particular, there is a hypothesis that inflammation, affecting endothelial cells (EC), stimulates the development and evolution of atherosclerosis, and also causes the occurrence of acute thrombotic complications. At the same time, inflammation can be not only a cause, but also a consequence of CHD. In this case, it first covers the myocardium and only then becomes systemic. The endothelium plays an undeniably important role in the regulation of inflammatory reactions.
The long-term impact of triggering pathophysiological stimuli on endothelial cells is the trigger for its proinflammatory activation with the development of endotheliitis, and in the future, endothelial dysfunction, which causes chronic dysregulation of the microcirculation of organs and tissues with the subsequent development of ischemic complications.
The article reveals the role of endotheliitis and endothelial dysfunction in the pathogenesis of CHD, demonstrates their clinical consequences, and also indicates the possibilities of enhancing pathogenetic treatment.
Keywords: coronary heart disease, angina pectoris, endotheliitis, endothelial dysfunction, L-arginine, L-carnitine.
Authors: Tashchuk V.K., MD, Professor, Head of the Department of Internal Medicine, Physical Rehabilitation and Sports Medicine, Bukovynian State Medical University, Chernivtsi, Ukraine.
Literature:
- Уніфікований клінічний протокол первинної, вторинної (спеціалізованої) та третинної (високоспеціалізованої) медичної допомоги (УКПМД) «Стабільна ішемічна хвороба серця»,2021
- Libby, P., Buring, J. E., Badimon, L., Hansson, G. K., Deanfield, J., Bittencourt, M. S., … Lewis, E. F. (2019). Atherosclerosis. Nature Reviews Disease Primers, 5(1). doi:10.1038/s41572-019-0106-z 10.1038/s41572-019-0106-z
- Ammirati, E.; Moroni, F.; Magnoni, M.; Camici, P.G. The role of T and B cells in human atherosclerosis and atherothrombosis. Clin. Exp. Immunol. 2015, 179, 173–187.
- В.О. Шумаков, Атеросклероз як клінічний прояв ендотеліальної дисфункції. Медична газета «Здоров’я України 21 сторіччя», тематичний номер «Кардіологія, Ревматологія, Кардіохірургія» 2020
- Gary H. Gibbons,Endothelial function as a determinant of vascular function and structure: A new therapeutic target, The American Journal of Cardiology, Volume 79, Issue 5, Supplement 1, 1997,Pages 3-8, ISSN 0002-9149, org/10.1016/S0002-9149(97)00122-7. № 1 (68), 2020 р
- О. А. Воробьева Терапевтические эффекты метаболитотропного кардиопротектора, содержащего L-аргинин и инозин, у больных пожилого и старческого возраста с ИБС. Патологія. 2012. 2. 98-101.
- Бабак, О.Я., Молодан, В.І. Просоленко, К.О.Железнякова, Н.М.Андреева, А.О.Зелена, І.І. Оптимізація діагностики функціонального стану ендотелію у хворих на гіпертонічну хворобу у поєднанні з ожирінням, методичні рекомендації 2014. repo.knmu.edu.ua/handle/123456789/9992
- Кравчун, П., Шелест, М., Ковальова, Ю., Шелест, Б., & Риндіна, Н. (2013). Особливості змін маркерів запалення у хворих на ішемічну хворобу серця з ожирінням. Медицина сьогодні і завтра, 59(2), 38-42. msz.knmu.edu.ua/article/view/145.
- В.І. Денисюк, О.В. Ковальчук, О.В. Денисюк. Ендотеліальна функція судин при ішемічній хворобі серця у поєднанні з артеріальною гіпертензією Практична ангіологія. 2008 6(17).
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; Maestrini, V.; Mancone, M.; Chilian, W.M.; Fedele, F. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. doi.org/10.3390/ijms21218118
- Аксьонов Є. В. Ендотеліальна дисфункція та шляхи її профілактики при проведенні рентгенендоваскулярних процедур по реканалізації коронарних артерій. Український журнал медицини, біології та спорту – Том 4, № 5 (21) doi: 10.26693/jmbs04.05.102
- Wang K, Zuo G, Zheng L, Zhang C, Wang D, Cao Z, et al. Effects of tirofiban on platelet activation and endothelial function in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cell Biochem Biophys. 2015; 71(1): 135-42. PMID: 25123839. DOI: 10.1007/s12013-014-0173-4
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kohno, H.; Hasegawa, A.; Toshima, S.; Amaki, T.; Kurabayashi, M.; Nagai, R.; Suzuki, T.; Amaki, T.; Nagai, R.; et al. Diagnostic implications of circulating oxidized low density lipoprotein levels as a biochemical risk marker of coronary artery disease. Clin. Biochem. 2002, 35, 347–353. [Google Scholar] [CrossRef]
- Mehta, J.L.; Li, D. Identification, regulation and function of a novel lectin-like oxidized low-density lipoprotein receptor. J. Am. Coll. Cardiol. 2002, 39, 1429–1435. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Masaki, T.; Sawamura, T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol. Ther. 2002, 95, 89–100. [Google Scholar] [CrossRef]
- Prinzmetal, M.; Kennarner, R.; Merliss, B.; Wads, T.; Bor, N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Maseri, A. Mechanisms of myocardial ischemia. Cardiovasc. Drugs Ther. 1990, 4 (Suppl. 4), 827–831. [Google Scholar] [CrossRef]
- Wu, N.; Li, W.; Shu, W.; Lv, Y.; Jia, D. Inhibition of Rho-kinase by fasudil restores the cardioprotection of ischemic postconditioninng in hypercholesterolemic rat heart. Mol. Med. Rep. 2014, 10, 2517–2524. [Google Scholar] [CrossRef][Green Version]
- Liuzzo, G.; Biasucci, L.M.; Gallimore, J.R.; Grillo, R.L.; Rebuzzi, A.G.; Pepys, M.B.; Maseri, A. The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N. Engl. J. Med. 1994, 331, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Mollace, V.; Gliozzi, M.; Musolino, V.; Carresi, C.; Muscoli, S.; Mollace, R.; Tavernese, A.; Gratteri, S.; Palma, E.; Morabito, C.; et al. Oxidized LDL attenuates protective autophagy and induces apoptotic cell death of endothelial cells: Role of oxidative stress and LOX-1 receptor expression. Int. J. Cardiol. 2015, 184, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Medina J, Muñoz M, Rodriguez C, Sanchez A, Contreras C, Carballido-Rodríguez J, Prieto D. Endothelial Dysfunction: An Intermediate Clinical Feature between Urolithiasis and Cardiovascular Diseases. Int J Mol Sci. 2022 Jan 14;23(2):912. doi: 10.3390/ijms23020912. PMID: 35055099; PMCID: PMC8778796
- Hattori Y, Hattori K, Machida T, Matsuda N. Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochem Pharmacol. 2022 Mar;197:114909. doi: 10.1016/j.bcp.2022.114909. Epub 2022 Jan 10. PMID: 35021044; PMCID: PMC8743392.
- Busse, I. Fleming, Vascular endothelium and blood flow, Handb. Exp. Pharmacol. 176 (pt 2) (2006) 43–78.
- Godo, H. Shimokawa, Endothelial functions, Arterioscler. Thromb. Vasc. Biol. 37 (9) (2017) e108–e114.
- Krüger-Genge, A. Blocki, R.-P. Franke, F. Jung, Vascular endothelial cell biology: an update, Int. J. Mol. Sci. 20 (2019) 4411.
- Davey MP, Martin TM, Planck SR, Lee J, Zamora D, Rosenbaum JT: Human endothelial cells express NOD2/CARD15 and increase IL-6 secretion in response to muramyl dipeptide. Microvasc Res 2006, 71(2):103–107
- Krausgruber T, Fortelny N, Fife-Gernedl V et al.Structural cells are key regulators of organ-specific immune responses // Nature. 2020;583(7815):296–302. Doi: 10.1038/s41586-020-2424-4.
- Armingol E, Officer A, Harismendy O, Lewis NE.Deciphering cell-cell interactions and communication from gene expression // Nat. Rev. Genet. 2021;22(2):71–88. Doi: 10.1038/s41576-020-00292-x.
- Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O. NF-κB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front Immunol. 2020 Dec 10;11:598444. doi: 10.3389/fimmu.2020.598444. PMID: 33362782; PMCID: PMC7759159.
- Приходько-Дибська К. COVID‑19: результати аутопсії [Електронний ресурс] / Режим доступу: www.umj.com.ua/article/183365/covid‑19-rezultati-autopsiyi
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020 May 2;395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020 Apr 21. PMID: 32325026; PMCID: PMC7172722.
- Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol. 2008 Jul-Aug;23(4):235-40. doi: 10.1080/08820530802111010. PMID: 18584561.
- Chudek J. Adipose tissue, inflammation and endothelial dysfunction / J. Chudek, A. Wiecek // Pharmacological Reports. – 2006. – Vol. 58, Suppl. – P. 81-88
- Versari D. Endothelial dysfunction as a target for preventation of cardiovascular disease / D. Versari, E. Daghini, A. Virdis // Diabetes Care. – 2009. – Vol. 32. – P. 314-321.
- Т.О. Проскура.Потенційні органи-мішені постковідного синдрому. ЖУРНАЛ НЕВРОЛОГІЇ ім. Б.М. Маньковського’ 2021, ТОМ 9, № 1-2 neuroscience.com.ua/index.php/journal/article/download/372/303/
- Becker RC. COVID-19-associated vasculitis and vasculopathy // J. Thromb. Thrombolysis. 2020;50(3):499–511.
- McGonagle D, Bridgewood C, Ramanan AV, Meaney JFM, Watad A. COVID-19 vasculitis and novel vasculitis mimics // Lancet Rheumatol. 2021;3(3):e224–e233.
- Seeßle J, Waterboer T, Hippchen T, Simon J, Kirchner M, Lim A, Müller B, Merle U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin Infect Dis. 2022 Apr 9;74(7):1191-1198. doi: 10.1093/cid/ciab611. PMID: 34223884; PMCID: PMC8394862.
- Doykov I, Hällqvist J, Gilmour KC, Grandjean L, Mills K, Heywood WE. ‘The long tail of Covid-19’ – The detection of a prolonged inflammatory response after a SARS-CoV-2 infection in asymptomatic and mildly affected patients. F1000Res. 2020 Nov 19;9:1349. doi: 10.12688/f1000research.27287.2. PMID: 33391730; PMCID: PMC7745182.
- Fogarty H, Karampini E, O’Donnell AS, Ward SE, O’Sullivan JM, O’Donnell JS; Irish COVID-19 Vasculopathy Study (iCVS) investigators. Persistent endotheliopathy in the pathogenesis of long COVID syndrome – Reply to comment from von Meijenfeldt et al. J Thromb Haemost. 2022 Jan;20(1):270-271. doi: 10.1111/jth.15578. Epub 2021 Nov 22. PMID: 34738307; PMCID: PMC8646468.
- Loktionova IL, Pokrovskiy MV, Ragulina VA, Titareva LV, Denisuk ТА, Stupakova EV,Sytnik МV, Saroyan KV, Losenok SА. The status of vascular endotheliumfunction in infectious diseases of various etiologies // Nauchnye vedomosti, Seriya Medicina. Farmaciya. 2012. No 4 (123);17/1: 20–31
- Kühnl A, Rien C, Spengler K, Kryeziu N, Sauerbrei A,Heller R, Henke A. Characterization of coxsackievirus B3replication in human umbilical vein endothelial cells // Med Microbiol Immunol. 2014;203(4):217–229. Doi: 10.1007/s00430-014-0333-6. PMID: 24615265.
- Han T, Lai Y, Jiang Y, Liu X, Li D. Influenza A virus infects pulmonary microvascular endothelial cells leading tomicrovascular leakage and release of pro-inflammatory cytokines // Peer J. 2021;(9):e11892. Doi: 10.7717/peerj.11892.PMID: 34414033; PMCID: PMC8344683.
- Han T, Lai Y, Jiang Y, Liu X, Li D. Influenza A virus infects pulmonary microvascular endothelial cells leading to microvascular leakage and release of pro-inflammatory cytokines // Peer J. 2021;(9):e11892. Doi: 10.7717/peerj.11892.PMID: 34414033; PMCID: PMC8344683.
- Herold S, Becker C, Ridge KM, Budinger GR. Influenza virus-induced lung injury: pathogenesis and implications for treatment // Eur Respir J. 2015;45(5):1463–1478. Doi:10.1183/09031936.00186214. PMID: 25792631.
- De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2106-13. doi: 10.1161/01.ATV.0000181743.28028.57. Epub 2005 Aug 11. PMID: 16100037.
- Ryoo, S., Berkowitz, D. E., & Lim, H. K. (2011). Endothelial arginase II and atherosclerosis. Korean Journal of Anesthesiology, 61(1), 3-11.
- В. Курята, Ю. С. Кушнір, Т. О. Віхрова. Медицина невідкладних станів. 2019. 3. 45-50. nbuv.gov.ua/UJRN/Medns_2019_3_9.
- Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function. Biomedicines. 2020; 8(8):277. doi.org/10.3390/biomedicines8080277
- Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997 Nov 18;96(10):3390-5. doi: 10.1161/01.cir.96.10.3390. PMID: 9396432
- Orsini E, Zito GB. Matching pathophysiology and evidence-based medicine for optimal management of ischemic heart disease. J Cardiovasc Med (Hagerstown). 2010;11(6):469-479
- В.О. Шумаков, Можливі шляхи ефективної енергетичної підтримки міокарда. Медична газета «Здоров’я України 21 сторіччя», № 5-6. 2022
- Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Аргінін de novo та утворення оксиду азоту в хворобливих станах. Am. J. Physiol. ендокринол. Метаб. 2012 рік; 303 :E1177–E1189. doi: 10.1152/ajpendo.00284.2012
- Palloshi A, Fragasso G, Piatti P, Monti LD, Setola E, Valsecchi G, Galluccio E, Chierchia SL, Margonato A. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am J Cardiol. 2004 Apr 1;93(7):933-5. doi: 10.1016/j.amjcard.2003.12.040. PMID: 15050504.
- Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, Celermajer DS. Oral L-arginine improves endothelium-dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Atherosclerosis. 1997 Mar 21;129(2):261-9. doi: 10.1016/s0021-9150(96)06044-3. PMID: 9105569.
- Maxwell AJ, Zapien MP, Pearce GL, MacCallum G, Stone PH. Randomized trial of a medical food for the dietary management of chronic, stable angina. J Am Coll Cardiol. 2002 Jan 2;39(1):37-45. doi: 10.1016/s0735-1097(01)01708-9. PMID: 11755284.
- Ceremuzynski L., Chamiec T., Herbaczynska-Cedro K. Effect of supplemental oral L-arginine on exercise capacity in patients with stable angina pectoris. Am. J. Cardiol. 1997;80:331–333. doi: 10.1016/S0002-9149(97)00354-8. [PubMed] [CrossRef] [Google Scholar] [Ref list]
- Tripathi P, Misra MK. Therapeutic role of L-arginine on free radical scavenging system in ischemic heart diseases. Indian J Biochem Biophys. 2009 Dec;46(6):498-502. PMID: 20361713.
- Yin, W. H., Chen, J. W., Tsai, C., Chiang, M. C., Young, M. S., & Lin, S. J. (2005). L-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clinical Nutrition, 24(6), 988-997.
- Schneider, J.Y., Rothmann, S., Schröder, F. et al. Effects of chronic oral L-arginine administration on the L-arginine/NO pathway in patients with peripheral arterial occlusive disease or coronary artery disease: L-Arginine prevents renal loss of nitrite, the major NO reservoir. Amino Acids 47, 1961–1974 (2015). org/10.1007/s00726-015-2031-0
- Song X, Qu H, Yang Z, Rong J, Cai W, Zhou H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2017;2017:6274854. doi: 10.1155/2017/6274854. Epub 2017 Apr 13. PMID: 28497060; PMCID: PMC5406747.
- В.О, Шумаков- Ефективність терапії пацієнтів із ішемічною хворобою серця з застосуванням фіксованої комбінації L-аргініну та L-карнітину з точки зору доказової медицини. Огляд міжнародних наукових джерел. Ukrainian Medical Journal.2021.143. 10.32471/umj.1680-3051.143.207515
- В.К. Тащук Медична газета «Здоров’я України 21 сторіччя», тематичний номер «Кардіологія, Ревматологія, Кардіохірургія» 2020
- DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O’Keefe JH. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc. 2013 Jun;88(6):544-51. doi: 10.1016/j.mayocp.2013.02.007. Epub 2013 Apr 15. PMID: 23597877.
- Askarpour M, Hadi A, Miraghajani M, Symonds ME, Sheikhi A, Ghaedi E. Beneficial effects of l-carnitine supplementation for weight management in overweight and obese adults: An updated systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol Res. 2020 Jan;151:104554. doi: 10.1016/j.phrs.2019.104554. Epub 2019 Nov 17. PMID: 31743774.
- І.О. Мітюряєва – Корнійко Імуномодулюючі можливості l-карнітіну – іноваційне медикаментозне супроводження терапії інфекційногопроцесу. Міжнародний журнал педіатрії, акушерства та гінекології Липень/Вересень 2018 Том 12 №3
- Koc A, Ozkan T, Karabay AZ, Sunguroglu A, Aktan F. Effect of L-carnitine on the synthesis of nitric oxide in RAW 264·7 murine macrophage cell line. Cell Biochem Funct. 2011 Dec;29(8):679-85. doi: 10.1002/cbf.1807. Epub 2011 Oct 19. PMID: 22012571.
- SCORE2 risk prediction algorithms:new models to estimate 10-year risk of cardiovascular disease in Europe European Heart Journal (2021) 00, 1–16 CLINICAL RESEARCH doi:10.1093/eurheartj/ehab309