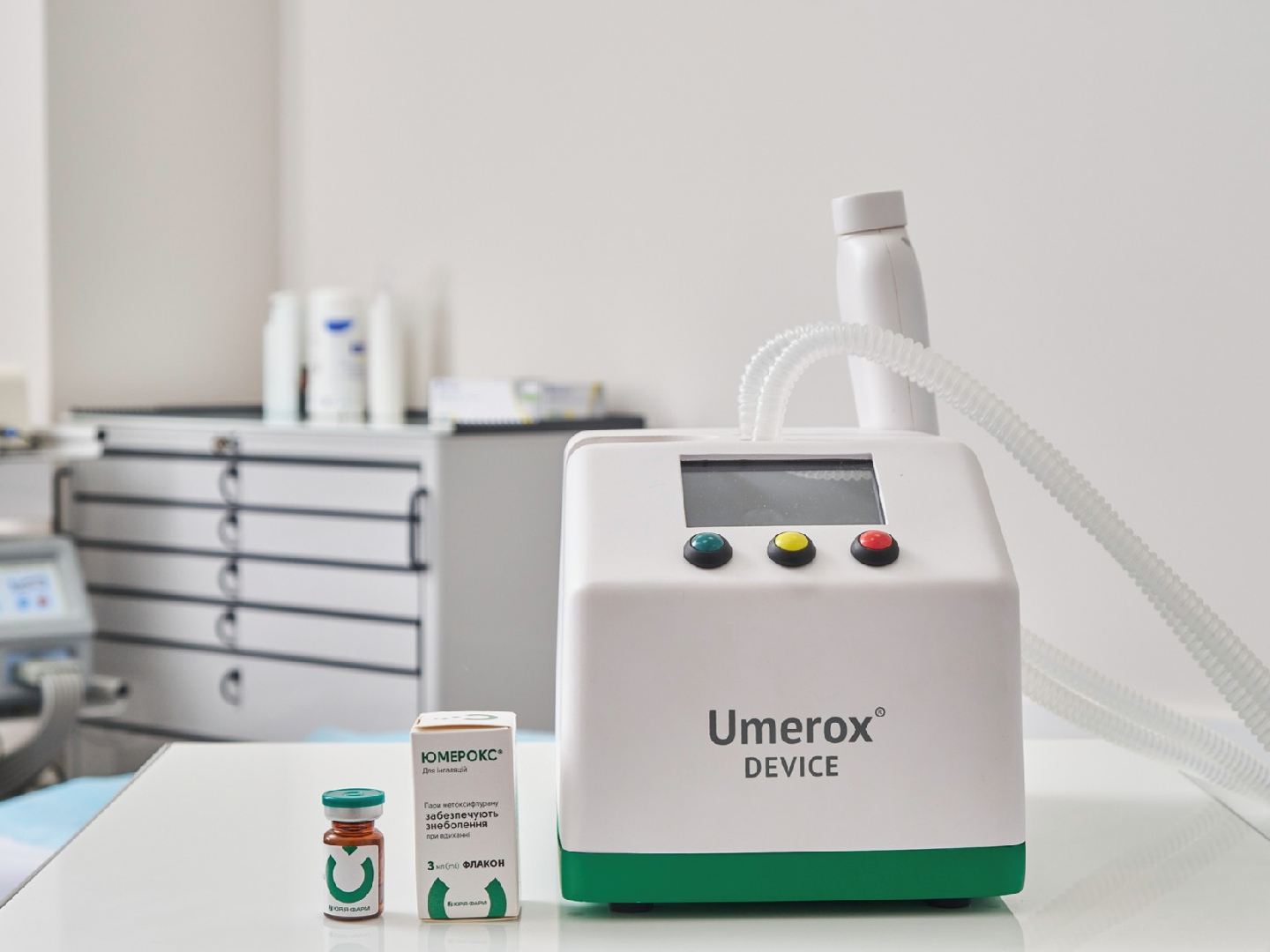
The use of inhaled analgesic Umerox (methoxyflurane) in a surgical inpatient setting
Abstract. The issue of effective and accessible pain relief for surgical patients, including those with combat injuries and in resource-limited settings, remains highly relevant, particularly in the context of armed conflict. Since the onset of the full-scale invasion of Ukraine, the number of patients with mine-explosive injuries has significantly increased. Most of these patients are admitted to surgical departments for repeated interventions, wound dressing changes, and NPWT (Negative Pressure Wound Therapy) system replacements – procedures frequently associated with severe pain.
Since 2022, inhalation analgesia with methoxyflurane (Umerox) has become available in Ukraine for the relief of pain during painful medical procedures. The aim of this study is to evaluate the effectiveness and safety of inhaled methoxyflurane (Umerox) during surgical procedures in patients with wounds managed by the open method.
Introduction. One of the pressing clinical challenges in surgical practice is the insufficient level of analgesia during painful procedures. Even when standard analgesic protocols are followed, some patients continue to experience significant pain and anxiety, which complicates the procedure, reduces treatment tolerance, and negatively affects the overall recovery process. In such cases, the search for effective, safe, and easy-to-administer analgesic options becomes especially relevant – particularly those that can complement standard methods within a multimodal pain management approach. One such option is the inhaled analgesic methoxyflurane.
Keywords: procedural analgesia, methoxyflurane, inhalation analgesic.
Authors:
- Prystupiuk Maksym, Kyiv City Clinical Hospital № 4.
Literature:
- Porter KM, Dayan AD, Dickerson S, Middleton PM. The role of inhaled methoxyflurane in acute pain management. Open Access Emergency Medicine [Internet]. 2018 Oct;Volume 10:149–64. Available from: dx.doi.org/10.2147/oaem.s181222
- Australian product information – Penthrox® (Methoxyflurane) inhalation [Internet]. 1993 [Last update 2 Aug 2023]. Available from: ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=pi&q=methoxyflurane
- New Zealand Datasheet for Penthrox [Internet]. 2002. [Last update 17 Jan 2020]. Available from: medsafe.govt.nz/profs/datasheet/p/penthroxinh.pdf
- Medical Developments International. Penthrox (methoxyflurane) inhalation product information. medicaldev.com/wp-content/uploads/2017/03/Penthrox-PI-13-Leaflet.pdf (2019, accessed 24 August 2020)
- Coffey F, Wright J, Hartshorn S, Hunt P, Locker T, Mirza K, et al. STOP!: a randomised, double-blind, placebo-controlled study of the efficacy and safety of methoxyflurane for the treatment of acute pain. Emergency Medicine Journal [Internet]. 2014 Apr 17;31(8):613–8. Available from: dx.doi.org/10.1136/emermed-2013-202909
- Fabbri A, Carpinteri G, Ruggiano G, Bonafede E, Sblendido A, Farina A, et al. Methoxyflurane Versus Standard of Care for Acute Trauma-Related Pain in the Emergency Setting: Protocol for a Randomised, Controlled Study in Italy (MEDITA). Advances in Therapy [Internet]. 2018 Nov 22;36(1):244–56. Available from: dx.doi.org/10.1007/s12325-018-0830-x
- Coffey F, Dissmann P, Mirza K, Lomax M. Methoxyflurane Analgesia in Adult Patients in the Emergency Department: A Subgroup Analysis of a Randomized, Double-blind, Placebo-controlled Study (STOP!). Adv Ther. 2016 Nov;33(11):2012-2031. doi: 10.1007/s12325-016-0405-7. Epub 2016 Aug 27. PMID: 27567918; PMCID: PMC5083764
- Gaskell AL, Jephcott CG, Smithells JR, et al. Self-administered methoxyflurane for procedural analgesia: experience in a tertiary Australasian centre. Anaesthesia 2016; 71: 417–423
- Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003 Sep;105(1-2):151-7. doi: 10.1016/s0304-3959(03)00176-3. PMID: 14499431
- Umana E, Kelliher JH, Blom CJ, et al. Inhaled methoxyflurane for the reduction of acute anterior shoulder dislocation in the emergency department. CJEM 2019; 21: 468–472
- Finkelstein S, Oliogu E, Yee A, Milton L, Rivlin L, Henry P, Behroozian T, Chow E, Finkelstein J. Literature review on the use of methoxyflurane in the management of pain in cancer-related procedures. Support Care Cancer. 2023 Mar 24;31(4):232. doi: 10.1007/s00520-023-07694-7. PMID: 36961562
- Nguyen NQ, Burgess J, Debreceni TL, et al. Psychomotor and cognitive effects of 15-minute inhalation of methoxyflurane in healthy volunteers: implication for post-colonoscopy care. Endosc Int Open 2016; 4: E1171–E1177
- Ho SF, Ganti S, Omar E, et al. Comparison of inhaled methoxyflurane versus procedural sedation for manipulation and reduction of acute shoulder and elbow dislocation in the emergency department. Proc Singap Healthc. 2022;31
- Penthrox® summary of product characteristics. Available at: medicines.org.uk/emc/medicine/31391. Date accessed: June 26, 2019
- Nguyen NQ, Toscano L, Lawrence M, Moore J, Holloway RH, Bartholomeusz D, Lidums I, Tam W, Roberts-Thomson IC, Mahesh VN, Debreceni TL, Schoeman MN. Patient-controlled analgesia with inhaled methoxyflurane versus conventional endoscopist-provided sedation for colonoscopy: a randomized multicenter trial. Gastrointest Endosc. 2013 Dec;78(6):892-901. doi: 10.1016/j.gie.2013.05.023. Epub 2013 Jun 28. PMID: 23810328
- Ashburn MA. Burn pain: the management of procedure-related pain. J Burn Care Rehabil. 1995 May-Jun;16(3 Pt 2):365-71. doi: 10.1097/00004630-199505001-00006. PMID: 7642683
- Wasiak J, Mahar PD, Paul E, Menezes H, Spinks AB, Cleland H. Inhaled methoxyflurane for pain and anxiety relief during burn wound care procedures: an Australian case series. Int Wound J. 2014 Feb;11(1):74-8. doi: 10.1111/j.1742-481X.2012.01067.x. Epub 2012 Aug 27. PMID: 22925206; PMCID: PMC7950743
- Ministry of Health of Ukraine. Guidelines regarding the anesthesia of victims during the evacuation stages [Metodychni rekomendatsiyi shchodo znebolennya postrazhdalykh na etapakh evakuatsiyi] [Internet]. 2022 Available from: moz.gov.ua/uploads/7/37722-dn_1122_28_06_2022_dod.pdf.
- 21 квітня Міністерство охорони здоров’я України оприлюднило для громадського обговорення проєкт наказу відомства щодо затвердження 17-го випуску Державного формуляру лікарських засобів. Станом на червень 2025 даний випуск знаходиться на громадському обговоренні.